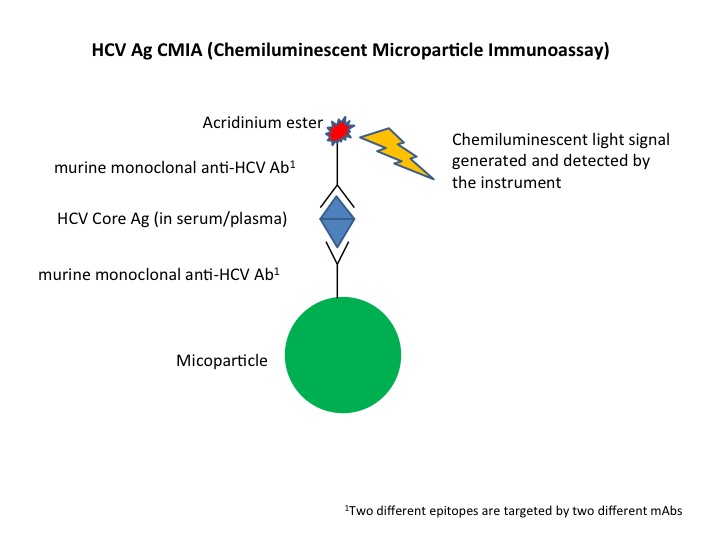
Diagnosis of active Hepatitis C virus (HCV) infection is currently performed using RNA testing in North America which is highly sensitive and specific but is associated with three major limitations: lability of RNA molecules, higher costs, and longer turn-around time as compared with commercially-available HCV core antigen testing. In the current study a new HCV core antigen assay product was evaluated for the diagnosis of HCV infection and its cost reducing potential. Ninety plasma specimens positive for HCV RNA along with 25 negative HCV specimens were used for HCV antigen assay.
Twenty-four specimens positive for a panel of agents were used for possible cross-reactivity. Sixty-four HCV antibody-positive specimens with negative HCV RNA and indeterminate HCV immunoblot results were also employed. In the first group 78/90 (86.6%) tested positive for HCV antigen with regression analysis showing no significant deviation from linearity. None of the prenatal specimens tested positive for HCV antigen. Non-specific reactions were not observed. In HCV antibody-indeterminate group, only 2/64 (3.1%) were antigen positive. In the last group, none of the HCV antibody very-low-positive specimens tested positive for HCV antigen. Both inter- and intra-run reproducibility of 100% were noted. The cost analysis showed a minimum of 52.13% reduction in costs associated with qualitative RNA testing.
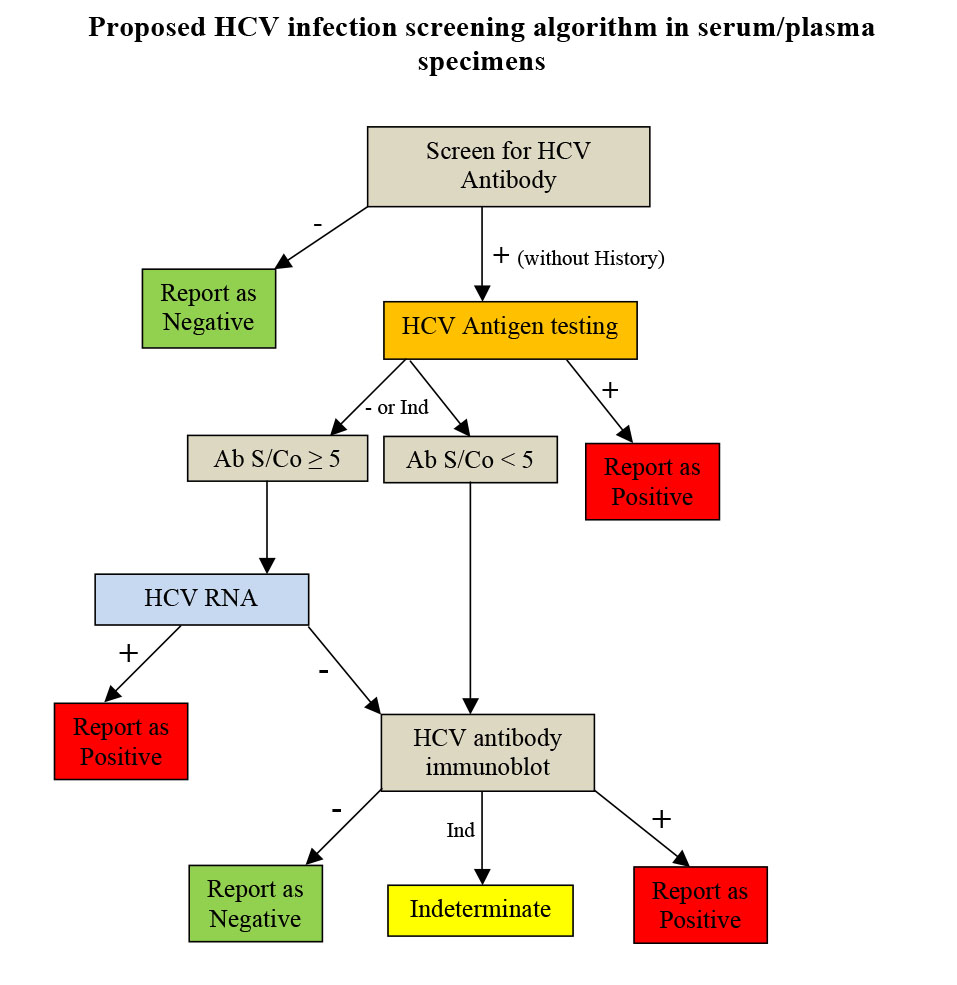
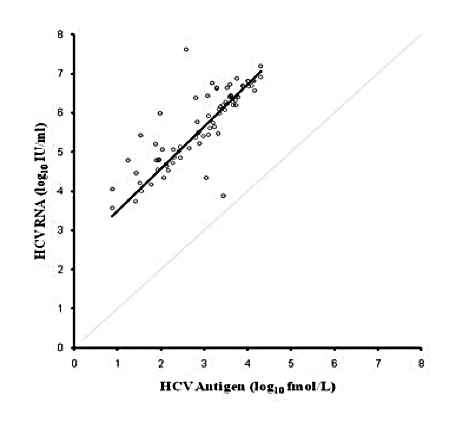
As shown in this study, HCV antigen and HCV RNA show similar kinetics trends, although RNA testing still remains more sensitive. HCV virions harbour a single copy of the RNA genome whereas HCV antigen could be found singly, in aggregates, associated with disrupted membranes, and complexed with antibodies which may lead to the observed suboptimal coefficient of determination, hence, not deemed reliable enough for treatment monitoring. The superior sensitivity of RNA testing could be due to the target amplification which does not occur in the case of antigen testing. That the presence of HCV antigen signifies an active HCV infection has a significant cost-, labour-, and turnaround time-reducing potential. Additionally, since antigen is much more stable than RNA, this obviates the need for strict collection, transport, and storage conditions. In this laboratory, based on the previous experience and in-house studies and also published literature, all serum specimens for HCV antibody are screened and those with a S/Co ratio ?5 are tested using qualitative HCV RNA testing and those with S/Co <5 are tested for HCV antibody by immunoblot as they are highly unlikely to be RNA-positive. In this article, incorporation of HCV antigen testing in the current algorithm is proposed to reduce the incurred costs and labour associated with HCV RNA testing and also to shorten the turn-around time. Considering the known fluctuating presence of HCV antigen, it is not suggested to replace initial screening of diagnostic specimens using HCV antibody by HCV antigen at this time. Furthermore, as it was shown in this study, RNA testing remains more sensitive than antigen testing and only should be attempted for diagnostic purposes whenever HCV antigen is negative.
Figure 2 summarizes such an algorithm. For recent exposures, immunocompromised patients (as shown in this study with two HIV-positive patients), and children younger than 18 months of age born to HCV-infected mothers, HCV core antigen testing should be tested in parallel with HCV antibody testing for screening and consequently, in case of a positive single HCV antigen only, HCV RNA testing should be attempted for confirmation.
Article Info
- Authors: Kamran Kadkhoda1,2,, Gerry Smart1
- Affiliation: 1, SerologySection, Cadham Provincial Laboratory, Winnipeg, Manitoba, Canada
2, Department of Medical Microbiology & Infectious Diseases, and Department of Immunology, Faculty of Medicine, University of Manitoba, Winnipeg, MB, Canada